Ellume COVID Test Kit, At Home COVID-19 Home Test Kit, Rapid Antigen Self Test, Results in 15 minutes to your free mobile app, FDA Emergency Use Authorization, 1 Pack
$15.66
681 in stock
The Ellume At-Home COVID-19 Test kit is an easy-to-use rapid antigen home test that detects an active COVID-19 infection in only 15 minutes without sending to a lab. It is suitable for adults and children ages 2+ years with or without symptoms. Convenient for individuals, businesses, schools, and events. Download the free Ellume COVID-19 Home Test App from the Apple or Google Play Store.
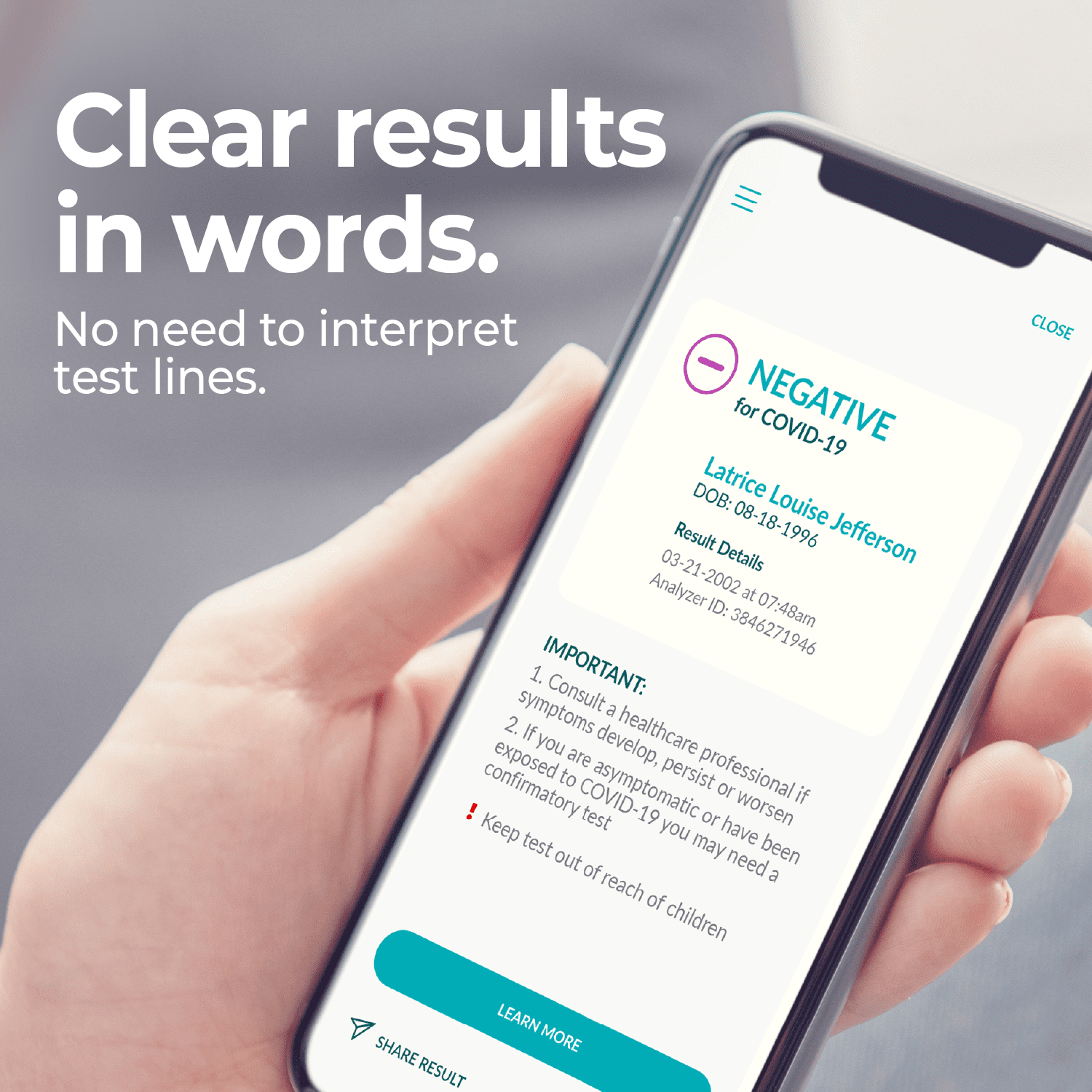
ELLUME COVID-19 HOME TEST APP. Our rapid test syncs directly to an app on your smartphone to guide you through the test with simple step-by-step instructions. The app clearly displays your negative or positive result in words and can be saved and shared with others as proof of result.
GROUNDBREAKING FLUORESCENT DETECTION TECHNOLOGY. The Ellume COVID-19 Home Test is the only home test to use innovative fluorescent detection technology, previously limited to healthcare professionals, to detect COVID-19 infection.
DETECTS COVID-19 VARIANTS. This test is designed to withstand mutations to the virus as it targets a protein in the virus (nucleocapsid protein), which remains largely unchanged in SARS-CoV-2 variants. Ongoing testing program monitors the test to confirm it covers the latest variants as they appear.
INSURANCE REIMBURSEMENT AVAILABLE. The Ellume COVID-19 Home Test may be fully reimbursable through your health insurer. Please contact your insurer directly for further information about reimbursement options and making a claim.
PATENTED USER-FRIENDLY NASAL SWAB. Uniquely designed swab comes with a child adapter to ensure safety & comfort when swabbing children, making this test perfect for children & adults. Suitable for ages 2 years and above.
Disclaimer: In the USA, this product has not been FDA cleared or approved; but has been authorized by FDA under an EUA. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens; and, the emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C.360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
| Dimensions | 5.9 × 3.8 × 2.2 in |
|---|
Only logged in customers who have purchased this product may leave a review.







Reviews
There are no reviews yet.